Oramed has completed an analysis of its U.S.-based Phase 3 oral insulin trial with significant lowering of A1C levels seen in patient subgroups
NEW YORK, May 15, 2023 /PRNewswire/ — Oramed Pharmaceuticals Inc. (Nasdaq: ORMP) (TASE: ORMP) (www.oramed.com) announced today that Hefei Tianhui Biotechnology Co. Ltd. (HTIT), a strategic partner of Oramed, has successfully completed its Phase 3 trials of oral insulin in type 2 diabetes in China under a differentiated study protocol. HTIT is now moving toward regulatory approval and has submitted the data to the National Medical Products Administration (NMPA, formerly the CFDA).
Oramed has recently completed an analysis of the data from its U.S.-based, Phase 3 trial, ORA-D-013-1, for the treatment of type 2 diabetes. This analysis found that subpopulations of patients with pooled specific parameters, such as body mass index (BMI), baseline HbA1c, age, gender and body weight, responded well to oral insulin. These subsets exhibited an over 1% placebo adjusted, statistically significant, reduction in HbA1c. The significant impact of baseline BMI on the responder group within the U.S. Phase 3 trial aligns with the positive data from the HTIT trial in China. The U.S. subpopulation and the Chinese general trial population shared a very similar baseline BMI.
“We are excited by our partner, HTIT’s, success and share in their excitement as they move one step closer to commercialization in China,” said Oramed Chief Executive Officer, Nadav Kidron. “Additionally, we are encouraged by the review of our Phase 3 data which found a strong correlation between certain parameters and the oral insulin’s efficacy. Based on these findings, Oramed is exploring ways to move forward with its oral insulin product,” Mr. Kidron added.
About Oramed Pharmaceuticals
Oramed Pharmaceuticals (Nasdaq: ORMP) (TASE: ORMP) is a platform technology pioneer in the field of oral delivery solutions for drugs currently delivered via injection. The Company’s novel Protein Oral Delivery (POD™) technology is designed to protect drug integrity and increase absorption. Oramed has offices in the United States and Israel. For more information, please visit www.oramed.com.
About the Methodology Used for US Phase 3 Data Analysis
Oramed applied two separate methodologies to retrospectively analyze the U.S. Phase 3 data*.
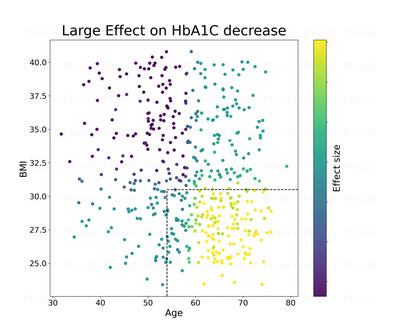
1. The Company applied causal-machine learning (ML) analysis to identify potential subgroup responders**. This method was successful in identifying multiple features responsible for the causal treatment effects. The two most important features were BMI and age as can be seen in the chart to the right.
Legend: X axis represents the age of patients in the trial
Y axis represents the BMI of patients in the trial
Bright colors (yellowish) represent large reduction in A1C for the treatment patients vs. placebo
Conclusion: Patients over 54 of age with BMI of less than 31 tend to have the largest reduction in A1c p-value lower than 0.002.
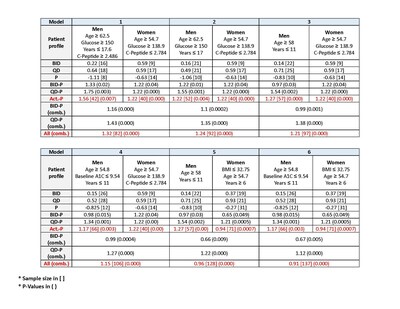
2. The analysis included a search for markers (demographic and clinical) that demonstrate the highest A1c improvement (measured between baseline and Visit 6) for each of the treatments compared with placebo for men, women, and both***. The results are displayed in the chart to the right.
* The data was compiled from 489 patients who completed both visit 6 (at 24 weeks) and all required laboratory data.
** Analysis was performed by PhaseV (https://phasevtrials.com/)
*** Analysis was performed by Panacea (https://www.panacea-ml.com/)
About HTIT
Hefei Tianhui Biotechnology Co., Ltd. (HTIT), has a state-of-the-art oral insulin manufacturing facility in Hefei, China. HTIT is a high-tech company focused on biopharmaceutical product manufacturing and R&D with an emphasis on the oral delivery of therapeutic macromolecules.
Forward-looking statements: This press release contains forward-looking statements. For example, we are using forward-looking statements when we discuss potential marketing approval by HTIT and commercialization in China, the potential safety and efficacy of oral insulin and the potential of Oramed to move forward with its oral insulin product. These forward-looking statements are based on the current expectations of the management of Oramed only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements, including the Company’s process to evaluate strategic options; the terms, timing, structure, benefits and costs of any strategic transaction and whether any transaction will be consummated at all; the impact of any strategic transaction on the Company; the outcomes of any litigation, regulatory proceedings, inquiries or investigations to which the Company may be subject; the ability to obtain financing or third-party approvals as needed; our ability to achieve the intended benefits of our strategic initiatives; the risks and uncertainties related to the progress, timing, cost, and results of clinical trials and product development programs; difficulties or delays in obtaining regulatory approval or patent protection for our product candidates; competition from other pharmaceutical or biotechnology companies; and our ability to obtain additional funding required to conduct our research, development and commercialization activities. In addition, the following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; delays or obstacles in launching our clinical trials; changes in legislation; inability to timely develop and introduce new technologies, products and applications; lack of validation of our technology as we progress further and lack of acceptance of our methods by the scientific community; inability to retain or attract key employees whose knowledge is essential to the development of our products; unforeseen scientific difficulties that may develop with our process; greater cost of final product than anticipated; loss of market share and pressure on pricing resulting from competition; laboratory results that do not translate to equally good results in real settings; our patents may not be sufficient; that products may harm recipients; and other factors discussed in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, which are on file with the Securities and Exchange Commission and in other filings that the Company makes with the Securities and Exchange Commission in the future. All of these factors could cause the actual results or performance of Oramed to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, Oramed undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
Company Contact:
Zach Herschfus+1-844-9-ORAMED
zach@oramed.com
Photo – https://mma.prnewswire.com/media/2077037/Oramed_Pharmaceuticals.jpg
Photo – https://mma.prnewswire.com/media/2077038/Oramed_Pharmaceuticals_2.jpg
Logo – https://mma.prnewswire.com/media/2077043/Oramed_Logo.jpg
 View original content to download multimedia:https://www.prnewswire.com/news-releases/oramed-announces-that-its-chinese-partner-htit-has-successfully-completed-a-phase-3-oral-insulin-clinical-trial-and-submitted-a-marketing-authorization-application-in-china-301824604.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/oramed-announces-that-its-chinese-partner-htit-has-successfully-completed-a-phase-3-oral-insulin-clinical-trial-and-submitted-a-marketing-authorization-application-in-china-301824604.html
SOURCE Oramed Pharmaceuticals Inc.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/oramed-announces-that-its-chinese-partner-htit-has-successfully-completed-a-phase-3-oral-insulin-clinical-trial-and-submitted-a-marketing-authorization-application-in-china-301824604.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/oramed-announces-that-its-chinese-partner-htit-has-successfully-completed-a-phase-3-oral-insulin-clinical-trial-and-submitted-a-marketing-authorization-application-in-china-301824604.html